Studyfinder and CATS IRB
Only those studies that have indicated in CATS IRB that they wish to use Studyfinder as a recruitment tool are included on the Studyfinder website. The website will pull trials once daily from CATS IRB if a modification to use Studyfinder for recruitment has been received and approved by the IRB. Investigators must follow the steps below to have their studies listed on Studyfinder. Studies will not be included on the site unless they are updated in CATS IRB.
How to update your study
To add Studyfinder as a recruitment option or to change study information displayed in Studyfinder:
Create a Modification or a Modification/CR in CATS IRB, and, after reaching the Studyfinder Recruitment page, answer the questions as follows:
Question 1
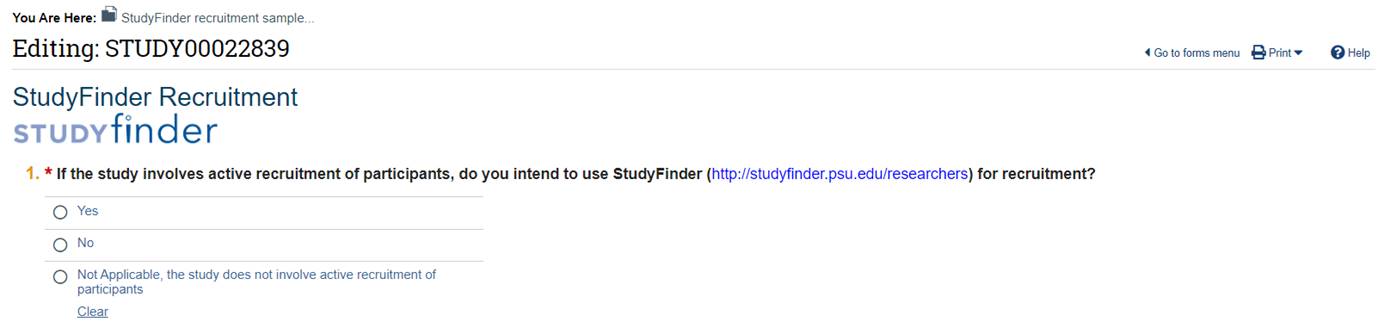
Choose “Yes” to include your study in Studyfinder once it has been approved by the IRB.
Questions 2 through 4
Questions 2 through 4 are pre-populated from other sources and are not editable on this form. To change the title or lay-language description of the study, do so on the Basic Information page.
The lay-language summary should include terms that are understandable to potential trial participants (fifth- or sixth-grade reading level). The example below shows the difference between a general CATS description and a good lay-language summary of the same study:
General CATS description: Interventions that increase activity levels have great potential to improve the public’s health. We propose developing and pilot testing games and organized noncompetitive sports for adults. We will modify sports using smaller fields and lighter balls to be less physically demanding, given that many adults lack the confidence to participate in strenuous sports. This pilot is being conducted to determine the feasibility of IRB#1861.
Good lay-language summary: Increased activity levels improve the public’s health. Volunteers will participate in games and organized noncompetitive sports for adults. We will modify sports using smaller fields and lighter balls to be less physically demanding.
Resources
Questions 5 and 6
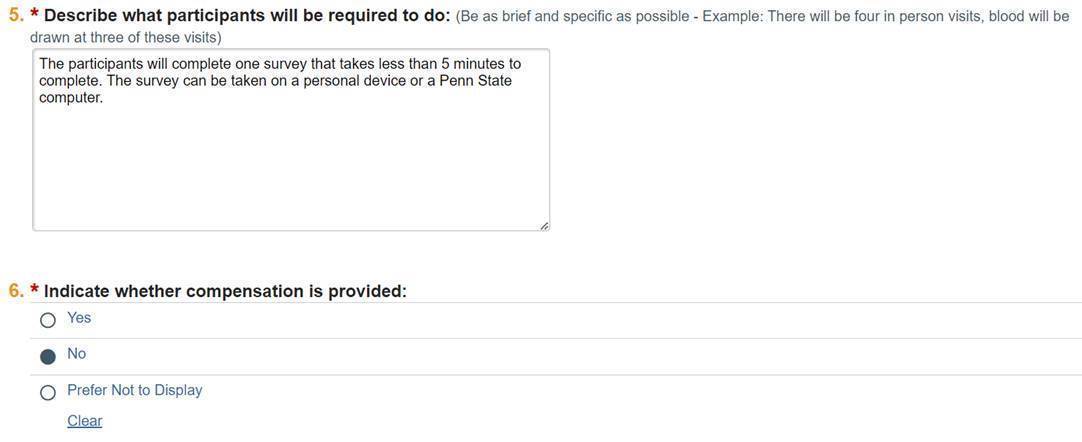
Give a brief and specific description of what participants will need to do. For example: "There will be four in person visits, blood will be drawn at three of these visits."
In question six, indicate whether compensation will be provided.
Question 7
Provide answers regarding the general age and gender of eligible participants, as well as if healthy volunteers are also needed. More detailed eligibility criteria (i.e. specific age ranges) can be provided in later questions.
Questions 8 to 10

Provide answers regarding the general age and gender of eligible participants, as well as if healthy volunteers are also needed. More detailed eligibility criteria (i.e. specific age ranges) can be provided in later questions.
Question 11
Choose up to three categories in which you wish your study to appear on the Studyfinder Search for a Study page.
Question 12
Choose all that apply to indicate the clinical trial phase of the study.
Questions 13 to 15
Provide specific inclusion and exclusion criteria and contact information. Examples might be detailed age ranges (such as 55 and older), having a particular condition, being a smoker or non-smoker, etc.
Fill out information for a contact person on the study team. The contact provided will receive an email notification from Studyfinder if someone expresses interest in this study. Verify that the email address is valid.
After completing the Studyfinder Recruitment page
If necessary, update the appropriate submission documents to include Studyfinder as a recruitment tool (e.g., protocol documents, recruitment materials, etc.) and submit the Modification or Modification/CR.
If using an external IRB
Studies using an external IRB can and should still opt to use Studyfinder for recruitment. This is done in the site smartform. Details can be found in CATS IRB's Help Center by clicking "Guides" and then opening "Job Aid for Researchers - External IRB Submissions."
How to remove Studyfinder as a recruitment option
If you are finished recruiting or no longer wish to use Studyfinder as a recruitment tool, create a Modification or a Modification/CR in CATS IRB.
Select “No” on the Studyfinder recruitment page.
If necessary, update the appropriate submission documents to remove Studyfinder as a recruitment tool (e.g., protocol documents, recruitment materials, etc.) and submit the Modification or Modification/CR.
What is the StudyFinder Volunteer Database?
Studyfinder is a web-based recruitment tool for Penn State researchers, managed and sponsored by Penn State Clinical Translational Science Institute (CTSI). It is available to all Penn State researchers actively recruiting participants or volunteers for studies. The volunteer database is managed by CTSI and enrolls volunteers who are interested in participating in research. If you choose to utilize this database as part of your recruitment efforts, your recruitment information will be shared on your behalf with eligible volunteers during the recruitment phase of your project. You will not be granted access to volunteer contact information unless they contact you for more information.
Is there template language to include in my IRB application?
This project will utilize the StudyFinder Volunteer Database to target eligible and interested research participants. Participants will be contacted by CTSI/College of Medicine (COM) Marketing via newsletter to share recruitment information for this study. Interested participants will reach out to our study contact to inquire about additional information, to determine eligibility, and to enroll in the study.
What are the requirements to utilize the StudyFinder Volunteer Database?
- The PI of your study must be a Penn State faculty member
- You must include language in your recruitment plan of your IRB protocol indicating that you plan to utilize the StudyFinder Volunteer Database as part of your recruitment efforts
- Your study must receive IRB approval
- Your study must be listed on StudyFinder
- You must complete the StudyFinder Volunteer Database application
What happens after my project is approved for use of the StudyFinder Volunteer Database?
The recruitment flyer and details you provide in your application will be used to identify eligible research participants in the database based upon inclusion criteria and/or matching their research interest(s) with your research topic(s). Brief information about your study as well as study contact information will be shared via newsletter every other week during the recruitment phase of your project. If you have reached your target number of participants prior to the recruitment end date you indicate in your application, you can contact Ellie Hogentogler at rhogentogler1@pennstatehealth.psu.edu to have your study removed from future newsletters. Similarly, if you need to extend your recruitment timeline, contact Ellie Hogentogler.
Who can I contact with questions?
If you have questions about the StudyFinder Volunteer Database, please contact ctsi@pennstatehealth.psu.edu.
StudyFinder Volunteer Database Principles:
- The StudyFinder Volunteer Database is a resource to be used to address high-priority research questions and oversight is provided by the Governance Committee
- If required, prioritization of projects will be the responsibility of the Governance Committee.
- Recruitment for your study to participants in the database will be granted for one year. Upon completion of that year, if the study continues to require recruitment assistance, investigators will need to submit a new request.
- Access to the Volunteer Database is provided for specific study and any change in the scope of the project must be approved by the IRB and the Governance Committee. Access is not provided for future unspecified uses.
Should multiple studies be competing for the same patients at the same time, priority will be given by a governance committee.